Diabetic wounds often lead to serious complications and may even lead to amputation, a global problem that affects more than 6% of the population. In Singapore, approximately four lower limb amputations are associated with non-healing diabetic wounds every day, with total amputation-related medical costs per patient reaching S$23,000. To address this challenge, researchers from the National University of Singapore (NUS) have developed two innovative microneedle technologies that effectively accelerate diabetic wound healing in preclinical models by retaining growth factor function and removing undesirable inflammatory compounds.
The two new technologies were developed by a team of scientists led by Assistant Professor Andy Tay from the Department of Biomedical Engineering and the Institute of Health Innovation and Technology at the School of Design and Engineering, National University of Singapore. Growth factors play a key role in the wound healing process, but in diabetic wounds, these growth factors are quickly broken down by proteases, resulting in slow wound recovery. At the same time, diabetic wounds are also accompanied by persistent high levels of inflammation.
"We wanted to solve both of these problems by using microneedles for both delivery and extraction," said Professor Andy Tay. "Microneedles are minimally invasive, can be precisely manufactured, and allow the active compounds to be painlessly injected directly into the wound. Microneedle patches are an excellent material for wound healing."
The results of two related studies were published online in the scientific journals Biomaterials and Advanced Functional Materials on July 4 and July 24, 2024, respectively, demonstrating the potential of this innovative approach in the treatment of various skin diseases such as psoriasis or chronic diabetic wounds.
Two unique methods to accelerate wound healing
Hydrogels are currently used on the market to deliver growth factors to wounds, but this approach has limited effectiveness in chronic wounds because the protease environment quickly degrades and inactivates the growth factors. The first approach developed by the NUS research team does not deliver growth factors directly, but instead increases the production of growth factors within the wound through aluminum sulfate microneedles (SUC-MNs). These microneedles can deliver the important immunomodulatory protein interleukin 4 (IL-4) to stimulate the production of growth factors in diabetic tissues, while aluminum sulfate can protect the growth factors from degradation. The researchers found that SUC-MNs significantly accelerated the rate of wound healing by two times compared to traditional treatments.
In the second approach, the NUS team explored new uses for microneedles to extract undesirable pro-inflammatory proteins and immune cells. They developed heparin-coated porous microneedles (HPMNs) to address the problem of persistent inflammation in skin wounds at the source. The research team demonstrated that HPMNs can effectively eliminate chemokines and monocytes at the wound site, resulting in a 50% reduction in tissue inflammation and a 90% reduction in wound area on the 14th day of treatment.
Innovative applications of microneedle technology
These preliminary findings highlight the potential of HPMNs as a promising strategy for treating inflammatory skin diseases. HPMNs have the unique advantage of being able to remove chemokines and inflammatory cells deep within the skin tissue, compared to existing treatments that only target surface inflammation. In the future, HPMNs could be further developed for personalized wound care and customized treatments for various inflammatory skin diseases, such as psoriasis.
Subsequent R&D plans
The development of SUC-MN and HPMN represents a significant advance in the field of wound healing and skin disease management. The team plans to conduct further research to explore the potential of this technology and bring it to market. For extractable microneedles, the team will use advanced technologies such as 3D printing to manufacture microneedles with more controlled pore sizes and incorporate antibacterial properties into the microneedles, as non-healing wounds in the clinic are often accompanied by infection. They also designed flexible microneedle patches to ensure that they can fit well into various tissue shapes.
"We are excited about the potential impact of our research and look forward to moving this technology towards clinical translation," said Professor Andy Tay. "The two approaches our team has developed will provide much-needed relief to patients with diabetic wounds, as well as the many patients suffering from skin diseases such as atopic dermatitis or psoriasis."

 English
English عربى
عربى Español
Español русский
русский 中文简体
中文简体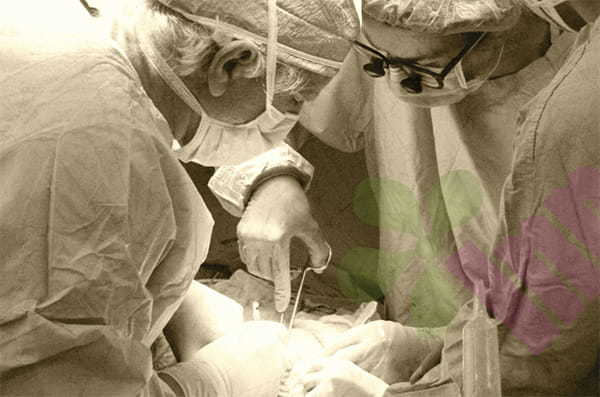













.jpg.png?imageView2/2/format/jp2)


